Material Transfer Agreements
A Material Transfer Agreement (MTA) is a contractual agreement that governs the transfer of tangible research material between two entities, when the receiving institution’s investigator intends to use the material in their own research project. MTAs address pre-existing and future intellectual property rights including ownership of materials and derivatives, liability issues that may result from the research, publication of research results, and limitation of use issues, and ensure that materials are shipped and handled in accordance with University biosafety standards. Examples of materials may include: assay materials, monoclonal antibodies, cell lines, mouse strains, plant varieties, technical data, software, confidential information, integrated circuit designs, blueprints, products, processes, devices, fabricated equipment, or any unique material. Material resulting from one's academic activities (such as a syllabus or course notes) would not usually fall under the parameters of an MTA. Purchased materials may require an MTA, depending on whether it is required by the vendor. MTAs that are required for purchases of goods or services are processed by the UNM Purchasing office. Those do not require a Streamlyne record and should be emailed to purchasingteam1@unm.edu, cc: sletten@unm.edu.
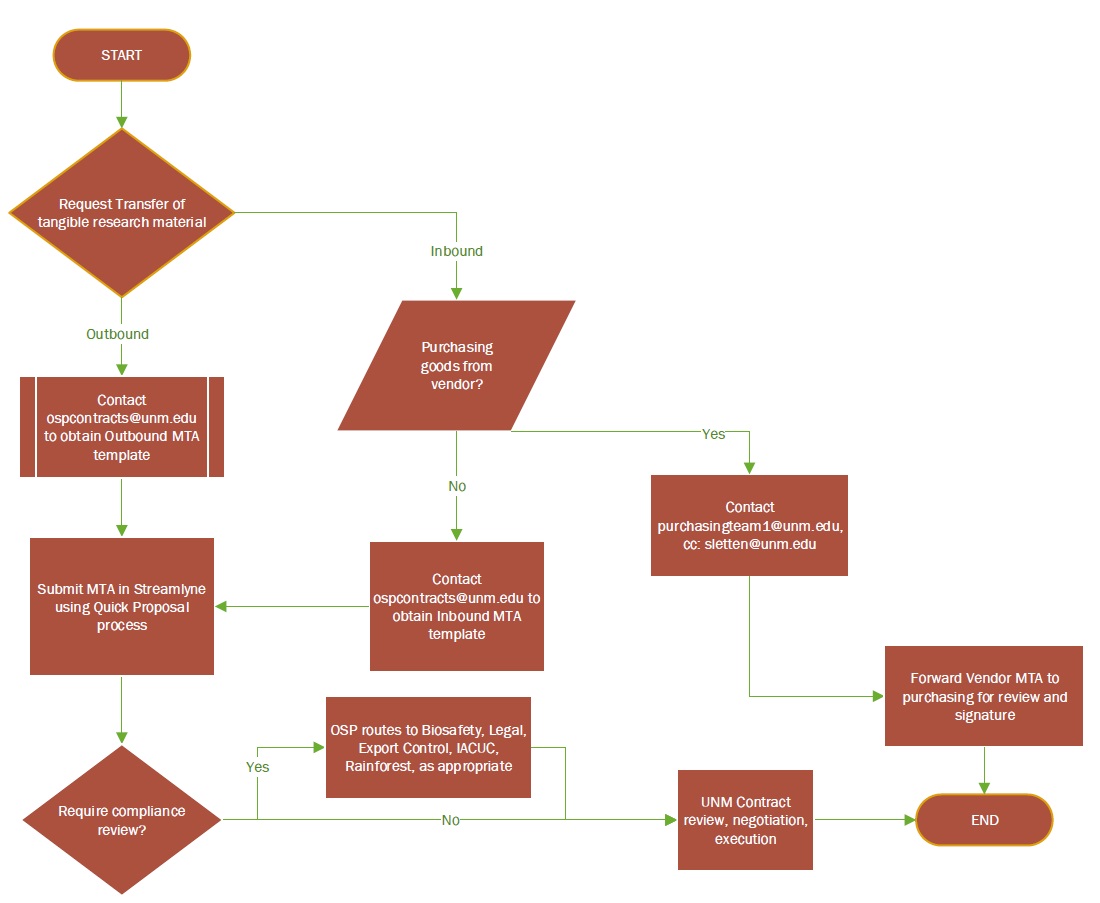
At the University of New Mexico, MTAs are required for any incoming or outgoing materials. Intra-UNM transfers of material are not subject to MTAs.
UNM Main Campus Material Transfer Agreement Process
Benefits of Material Transfer Agreements
MTAs provide UNM faculty and staff with many benefits:
- Define and protect rights to innovations, intellectual property rights, and commercialization resulting from material
- Rights to improvements to material and/or innovations (including patentable inventions) made using material
- Timely sharing of material/Control over the distribution of material
- Acknowledgment of UNM investigators in publications resulting from use of their material
- Ensuring that recipients exercise care in handling material
- Provide safeguards that recipients will follow relevant federal guidelines relating to recombinant DNA, protection of human subjects in research, and the use of animals
Execution of Material Transfer Agreements
The UNM Office of Sponsored Projects (OSP) provides templates for both Incoming transfers as well as Outgoing transfers. If you are interested in using any of the available templates, please reach out to OSP at ospcontracts@unm.edu. OSP will assist in identifying a template that best meets the needs of your project.
Instructions on how to submit an MTA for review and approval via the Streamlyne Quick Proposal process can be found here: https://streamlyne.unm.edu/quick-proposal-overview-june-2023.pdf
Upon submission of the MTA to OSP in Streamlyne, the agreement is reviewed by a Contract Officer and any relevant compliance office (e.g. biosafety, legal) before being signed by an institutional authorized representative.
When is Supplemental Compliance Review of MTAs Required?
Biosafety Review is required when working with:
Human Materials | Blood, blood components, primary cell lines, established cell lines, body fluids, and unfixed tissues or organs |
|---|---|
Non-Human Primate Materials | Blood, blood components, primary cell lines, established cell lines, body fluids, and unfixed tissues or organs |
Animal Materials | Blood, blood components, primary cell lines, established cell lines, body fluids, and unfixed tissues or organs |
Plant Materials | Biology Department (baseline data) |
Other Biological Agents and Toxins | Bacteria, Fungi, Parasites, Prions, Rickettsia, Virus, Viral Vectors, and Biotoxins |
Legal Review may be required when a contract or agreement includes language that UNM is not able to accept, in that case, OSP will negotiate the terms and conditions with the sponsor.
Export Control review may be required for materials and/or equipment that need to leave UNM’s custody when 1) a Project is under a Technology Control Plan (determined at time of contract start based on customer documentation and requirements), or 2) Export Controlled materials were purchased under the contract.
IACUC review may be required when animal materials are being transferred. OSP will verify IACUC approval prior to receipt of the materials and/or any work with the materials.
Rainforest Innovations review may be required if the material transfer involves invention disclosures or patent applications.
Shipping Policy and Training
UNM’s Biosafety Office provides research groups training on shipping of biological materials domestically and internationally.
For more information, please contact the appropriate office listed below.
UNM Contacts:
Main Campus OSP: ospcontracts@unm.edu
Purchasing: purchasingteam1@unm.edu; Brett Sletten- sletten@unm.edu
HSC Biosafety Office: tmuller@salud.unm.edu; VSeverns@salud.unm.edu
IBC submissions or administration: IBC@salud.unm.edu
Routine BioInventory Business: BioInventory@salud.unm.edu
